【Principles】
Vitamin B7 (Biotin) Test Kit used microbiology method to detect the volume of Biotin in food matrices. Biotin supplies the growth of Lactiplantibacillus plantarum. Its growth rate associated with the volume of Biotin within the sample. Take the Biotin medium (Lactiplantibacillus plantarum added), samples or Standards and add to the microwell. Incubate at 37℃ and avoid light exposure. Lactiplantibacillus plantarum will start growing until Biotin are consumed entirely. It takes 44-48 hours to complete incubation. Use microplate reader to read the sample turbidity with 610-630nm or 540-550nm filter. The volume of Biotin in sample can be determined by comparing the sample turbidity with the Standards.
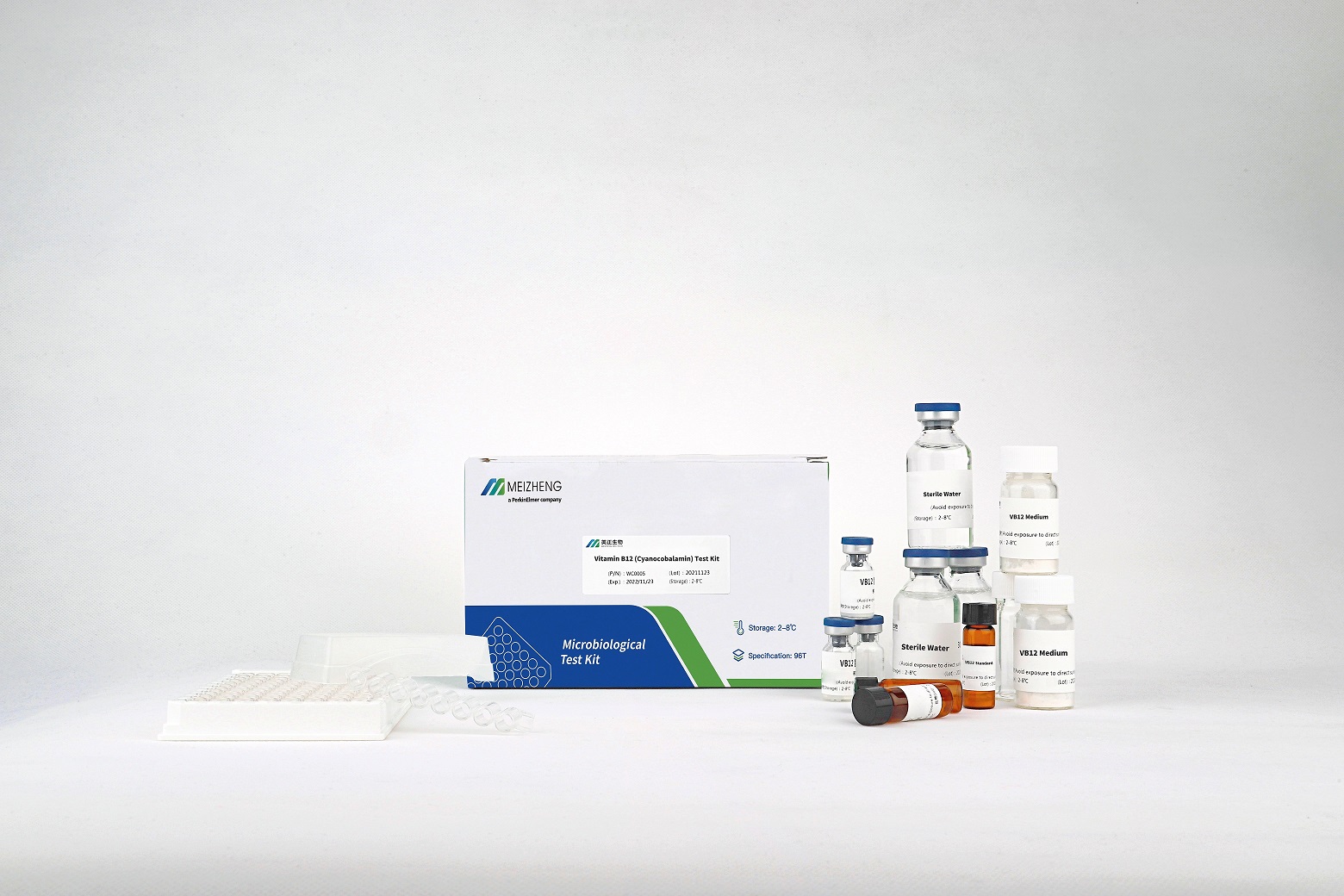
【Provided Materials and Reagent】
| Component | Qty |
| Microwell plate | 96T |
| Cover film | 3 |
| Plate rack | 1 |
| Reagent reservoir | 3 |
| Package Insert | 1 |
| Testing Report | 1 |
| Sterile water | 3 bottles |
| Biotin Medium | 3 bottles |
| Biotin Standards | 3 bottles |
| Biotin Assay Strain | 3 bottles |
【Materials Required But Not Provided】
- Devices
—- Clean bench
—- Microplate Reader (610-630nm or 530-540nm filter)
—- Water Bath: 95℃
—- Incubator: 37℃
—- Centrifuge (≥8000g)
—- Sterilized tips: 200μL, 1000μL, 10mL
—- Sterilized centrifuge tubes: 15mL, 50mL
—- Sterilized reaction tubes: 1.5mL / 2mL
—- Sterilized syringe and 0.22μm sterilized filter
—- Vortex
—- Sterilized tweezers
- Reagent for native Biotin detection
—- Sterile water
—- 5M NaOH: 20g NaOH dissolved in 100mL redistilled water
—- 0.1M NaOH: 0.4g NaOH dissolved in 100mL redistilled water
—- 3% H2SO4: Add 30mL H2SO4 (Sulfuric Acid) to 1000mL deionized water slowly with stirring and mix well for use
—- Taka-Diastase from Aspergillus oryzae
—- Citrate buffer (pH 4.5): weigh 1.5g C6H8O7·H2O (Citric acid monohydrate) in a 100mL beaker, add 50mL redistilled water and dissolve on a magnetic stirrer. Add 0.48g NaOH, adjust pH to 4.5 with 0.1M NaOH. Transfer the solution in a 100mL volumetric flask and fill up with redistilled water to 100mL. The buffer can be stored at 2-8℃ for 3 days
【Sample Preparation】
Sample Pretreatment
- Liquid samples with added Biotin (fruit juices, drinks)
- Add 1mL homogeneous sample to a 50mL sterilized centrifuge tube.
- Add 40mL sterile water or deionized water, vortex to mix well.
- Filter it with 0.22μm sterilized filter and collect the filtrate.
- Depends on the Biotin concentration range, dilute the filtrate further in 1.5mL or 2mL sterilized reaction tubes with sterile water from kit.
- Milk powder, baby food, bakery food and wheat flour with added Biotin
- Weigh out 1g (mL) homogeneous sample to a 50mL sterilized centrifuge tube.
- Add 40mL sterile water or deionized water, vortex to mix well. Extract in the 95℃-Water Bath for 30 minutes. Mix it every 10 minutes (make sure the centrifuge tube is sealed), then cool it down to 30℃ rapidly.
- Filter it with 0.22μm sterilized filter and collect the filtrate. Or centrifuge (≥8000g) it for 5 minutes, pipette the middle layer.
- Depends on the Biotin concentration range, dilute the filtrate further in 1.5mL or 2mL sterilized reaction tubes with sterile water from kit.
- Jelly with added Biotin
- Weigh out 20g jelly sample to a 100mL sterilized centrifuge tube.
- Add 30mL sterile water or deionized water, vortex to mix well. Extract in the 95℃-Water Bath for 30 minutes. Mix it every 10 minutes (make sure the centrifuge tube is sealed), then cool it down to 30℃ rapidly.
- Add sterile water or deionized water to 80mL, then pipette 4mL sample solution (equivalent to 1g sample) to a 50mL sterilized centrifuge tube. Then, add deionized water exactlyto 40mL, vortex to mix well.
- Filter it with 0.22μm sterilized filter and collect the filtrate.
- Depends on the Biotinconcentration range, dilute the filtrate further in 1.5mL or 2mL sterilized reaction tubes with sterile water from kit.
- Total content of Biotin(added and native) for dairy product and baby food
When testing the content of Biotin (native) in food, samples should be extracted with enzymatic treatment.
- Weigh out 1g (mL) homogeneous sample to a 50mL sterilized centrifuge tube.
- Add 20mL Citratebuffer (pH 4.5), vortex to mix well.
- Add 300mg Taka-Diastase, and incubate at 37℃ without light for 1 hour (vortex regularly). Then, add sterile water or deionized water to 40mL.
- Then, extractthe sample in the 95℃-Water Bath for 30 minutes. Mix it every 10 minutes (make sure the centrifuge tube is sealed), then cool it down to 30℃ rapidly.
- Filter it with 0.22μm sterilized filterand collect the filtrate. Or centrifuge (≥8000g) it for 5 minutes, pipette the middle layer.
- Depends on the Biotinconcentration range, dilute the filtrate further in 1.5mL or 2mL sterilized reaction tubes with sterile water from kit.
Note: When enzymatic treatment is required for sample preparation, a blank control is recommended to ensure that the enzyme reagent used does not contain Biotin.
- Total content of Biotin(added and native) for cereal
When testing the content of Biotin (native) in cereal, samples should be extracted with high pressure treatment under acid condition.
- Weigh out 1g homogeneous sample to a 50mL sterilized centrifuge tube.
- Add 20mL 3% H2SO4, vortexto mix well.
- Extractthe sample at 121℃ high pressure for 15 minutes, then cool it down to 30℃ rapidly.
- Adjust pH to 7 with 5M NaOH, then add sterile water or deionized water to 40mL.
- Filter it with 0.22μm sterilized filter and collect the filtrate.
- Depends on the Biotin concentration range, dilute the filtrate further in 1.5mL or 2mL sterilized reaction tubes with sterile water from kit.
Sample Dilution
- Dilution factor calculations
Using reasonable dilution factor can ensure the accuracy of the test when the sample concentration falls within the standard curve.
For example, for sample with 50μg/100g of Biotin, dilution factor can be calculated by taking the concentration of Biotin divide by Standard 2. Dilution factor = 50μg ÷ 0.24μg ≈ 208, choose 200 as dilution factor.
- Sample Dilution Procedure
- 1:9 (50μL sample + 450μL sterile water from kit)
- 1:9 (50μL of solution from a) + 450μL sterile water from kit)
- 1:1 (250μL of solution from b) + 250μL sterile water from kit)
Note: Mix well before each dilution. Dilute the samples fresh before usage. The result is the most accurate for the samples when diluting between the concentration of Standard 2 and Standard 3.
Precautions during preparation
- For samples with lower Biotin concentration, weigh 5g of samples instead (it should take into consideration when calculate the result).
- Lower concentration samples with darker color (darker color may affect the assay result)can be filtrated by Whatman 934-AH. Collect the filtrate for
- Samples are difficult to filter should be centrifuge (≥8000g, 5 minutes) first to remove the particles. Then, filter it with 0.22μm sterilized filter.
- If the samples needed to be diluted further for the extraction (dilution factor greater than 10), dilute them 10-fold or lower than 10-fold in gradient. At each gradient, ensure the samples are homogeneous (make sure to vortex well). If samples are not homogeneous, it will affect the result significantly. Dilution method should follow the steps of Sample Dilution.
- Prepare Standards and samples in triplicates.
- Unknown samples should dilute in two different gradients to make sure it falls within the standard curve.
- If samples are not being used immediately, store them at 4℃ and avoid light exposure. Only use them within the same day.