1. Aerobic plate count
The total number of colonies refers to the number of colonies grown per gram (per milliliter) of sample under certain conditions (such as aerobic conditions, nutrient conditions, pH, culture temperature and time, etc.). The determination of the total number of bacterial colonies is used to determine the degree of bacterial contamination and the hygienic quality of the food. It reflects whether the food meets the hygienic requirements during the production process, so as to make an appropriate hygienic evaluation of the tested sample. The total number of bacterial colonies marks the quality of food hygiene to a certain extent.
2. The risk of unqualified total number of colonies
Eating food with a total number of bacterial colonies exceeding the standard may cause symptoms such as acute poisoning, vomiting, diarrhea, and endanger human health and safety.
3. The reasons and countermeasures for the unqualified total number of colonies have just been analyzed
What are the reasons and solutions for the total number of colonies exceeding the standard? Below we will analyze them one by one from six aspects.
1. Personnel
The incorrect behavior of employees in all aspects of food production, transportation and sales may lead to the total number of bacterial colonies exceeding the standard, and the more common ones are:
1. The personnel in charge of cleaning and disinfection are not in place or do not understand the frequency and requirements of cleaning and disinfection, which may lead to poor sanitary conditions in the production environment, continuous use of production equipment without cleaning and disinfection, and poor cleaning or lax disinfection of production equipment. The occurrence of microbial retention and breeding causes food contamination, which in turn leads to the total number of bacterial colonies exceeding the standard.
2. The production operators do not operate according to the production requirements. If the personnel responsible for the sterilization process do not implement or understand the parameters and requirements of sterilization in place, it may lead to incomplete sterilization.
3. Staff training is not in place and lack of hygiene awareness. For example, cross-contamination occurs regardless of raw and cooked during processing, which leads to the total number of colonies exceeding the standard.
In my opinion, if you want to completely solve the human factor that causes the total number of colonies to exceed the standard, the way is to train, train, and retrain! Only through continuous training and improving employees’ hygiene awareness and operating standardization can we achieve the desired effect.
equipment
The capacity and status of equipment and facilities may also be associated with excess colony counts. Here we take sterilization equipment and packaging machines as examples.
For products that need to be sterilized after packaging, if the performance of the sterilization equipment is insufficient and the thermometer is not calibrated, resulting in incomplete sterilization, the total number of colonies may exceed the standard. For sterilization equipment, we can prevent the total number of colonies from exceeding the standard by doing thermal distribution and thermal penetration tests, verifying the performance of the sterilizer, using temperature recorders regularly, tracking changes in product temperature during the sterilization process, and calibrating thermometers.
Similarly, insufficient sealing performance of packaging machines may lead to product contamination during sterilization and subsequent storage. For the packaging machine, we can establish the performance verification of the packaging machine to ensure good sealing, and at the same time do a good job in the cleanliness of the in-package workshop and packaging machine to reduce the possibility of the total number of colonies exceeding the standard.
materials
The materials mainly include various raw and auxiliary materials, inner packaging materials, production water and ice, etc. If the original microbial content of various materials is high, there is still a risk of increasing the total number of colonies in the products in the later stage. Therefore, we should target the packaging materials for raw and auxiliary materials. Carry out evaluation, formulate certain acceptance requirements and carry out corresponding testing of raw and auxiliary materials and packaging materials, and do a good job in temperature and humidity control and environmental management during storage and processing.
In addition, the inner packaging material can also be sterilized by ultraviolet lamp or ozone before use. For production water and ice, the microbiological status can be verified on a weekly basis.
method
The method mainly refers to the rationality of the formulation of various microbial measures. For example, are the frequency and methods of cleaning and disinfection of equipment, environment, and personnel reasonable? Is the setting of the sterilization formula reasonable? Is the control of ambient temperature and humidity in the production process reasonable? Whether the conditions (such as cold chain) set in food storage and transportation are reasonable, etc.
In this case, the action we can take is to verify. By conducting microbiological experiments before and after, the data obtained are compared to confirm whether the method is feasible. In addition, we can also optimize the method by adjusting certain parameters and collecting experimental data to determine the most suitable method.
environment
Improper places in all aspects of transportation, storage, processing into finished products, and sales of raw and auxiliary materials may cause the total number of product colonies to exceed the standard. Such as improper sanitation of the packaging workshop, improper control of temperature and humidity in the production environment, improper location and facilities of the toilet in the waste room, etc.
For the environment, the measures we can take are to reasonably consider the layout of each place, strictly control the temperature and humidity of each link, and continue to maintain the hygiene of each place.
circulation
If it is detected that the total number of bacterial colonies in various foods exceeds the standard in the circulation process, the problem is most likely that the seller has not stored and placed the food in accordance with the regulations. For example, some supermarkets do not have low-temperature refrigeration facilities but sell food that needs to be refrigerated. The label of the food states that the storage condition is protected from light, but the seller places the food in direct sunlight, etc.
summary
(1) As a consumer, you should buy food that is within the shelf life, with complete packaging and normal shape. Don’t buy three no products. If it is cooked processed food, it needs to be cooked thoroughly and stored at the correct temperature. When discovering quality and safety problems in the purchased food, they should report or complain to the local consumer association or relevant regulatory authorities in a timely manner to safeguard their legitimate rights and interests.
(2) As a production enterprise, it should strictly control the raw materials of the products, so as not to purchase or use expired or stale raw materials. For the production and processing environment, it is necessary to distinguish between clean areas and non-clean areas, and do a good job of preventing rodents and insects. At the same time, it is necessary to strengthen the food safety awareness of food practitioners and improve the hygiene management requirements.
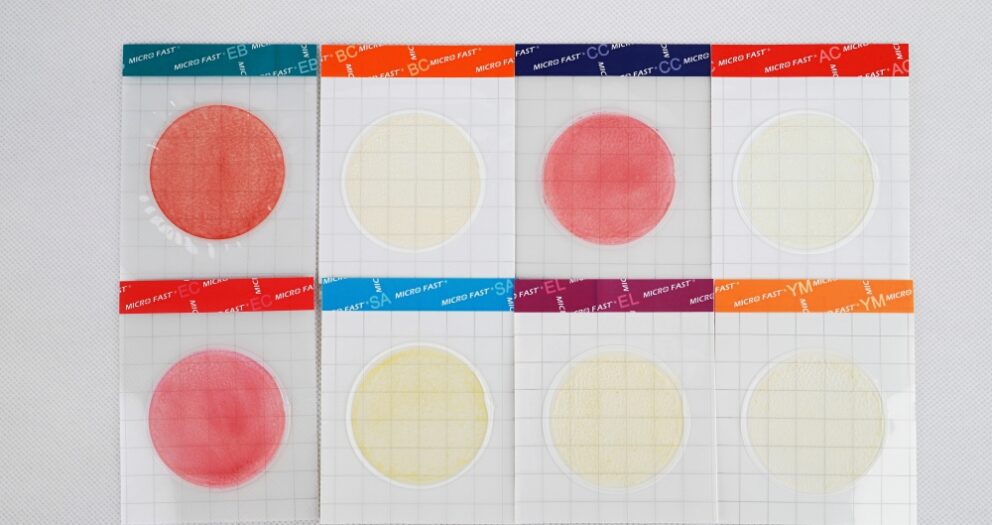
Microfast Aerobic plate counts can test aerobic easily.