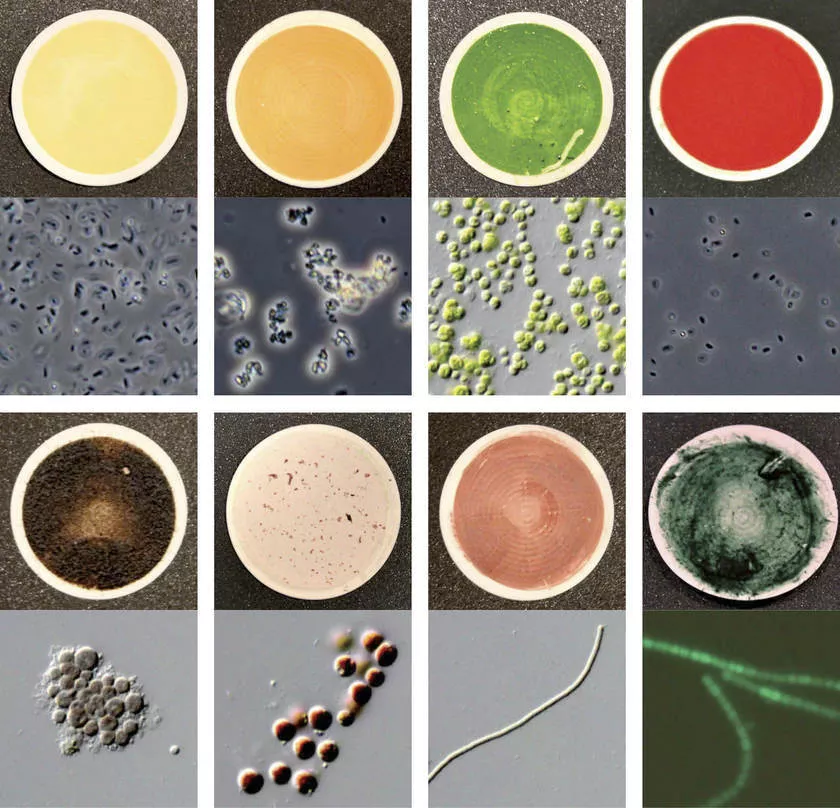
① Accuracy
The accuracy of the microbial quantitative test refers to the degree to which the test results of the alternative method are consistent with the test results of the pharmacopoeia method, and is usually expressed by the recovery rate (%) of microorganisms.
Accuracy verification method: prepare the bacterial suspension of the test bacteria, the concentration of the bacterial suspension should be selected as the highest concentration that can be accurately counted, and then serially diluted to a lower concentration (such as less than 10cfu/ml). For example, an alternative to the colony-counting plate method is to prepare high-strength bacterial suspensions at a concentration of 103 cfu/ml, serially diluted to 100 cfu/ml. For each test bacteria, at least 5 bacterial concentrations should be selected for accuracy confirmation, and the test results of the alternative method should not be less than 70% of the test results of the pharmacopoeia method. Appropriate statistical methods can also be used to show that the recovery rate of the alternative method is at least consistent with the pharmacopoeia method. . When the recovery of the alternative method is higher than that of the compendial method, it is necessary to combine the suitability of the specific sterility test method.
② Precision
The method of precision verification is: prepare the bacterial suspension of the test bacteria, the concentration of the tooth suspension should be selected as the highest concentration that can be accurately read, and then serially diluted to a lower concentration (such as less than 10cfu/ml). For each test bacteria, select at least 5 concentrations of the bacterial suspension for testing. At least 10 replicate tests should be performed for each concentration so that the standard deviation or relative standard deviation can be obtained by statistical analysis
③ Exclusive
Specificity verification should prove that when there is a certain amount of test bacteria in the sample, the test bacteria can be detected by the plate method, and the existence of the sample will not affect the results. When validating specificity, it should be possible to design experimental models that may give false positives to the alternative method to challenge the alternative method. When the alternative method can be quantified without relying on microorganisms to grow colonies or appear turbid (such as a quantitative method that does not require enrichment or can directly measure the number of bacteria in the range of 1 to 50 cfu), the above verification methods are even more important.
④ Quantitation limit
The method of quantification limit verification is: prepare 5 bacterial suspensions with different bacterial concentrations at the lower limit of the inspection range, and each bacterial suspension is tested no less than 5 times by the pharmacopoeia method and the alternative method, and the statistical method is used to compare and replace the The difference between the test results of the method and the results of the compendial method to evaluate the limit of quantification of the alternative method
⑤ Linearity and range
Linearity verification must cover the full range of concentrations that can be accurately measured. At least 5 concentrations should be selected for each strain of test bacteria, and each concentration should be measured at least 5 times. According to the above experimental data, take the test result as the dependent variable and the expected number of microorganisms in the sample as the independent variable to carry out the linear regression analysis, and calculate the correlation coefficient r. The correlation coefficient for the alternative method must not be lower than 0.95.
The scope of quantitative microbiological testing refers to the range of high and low-limit concentrations or quantities to which certain accuracy, precision and linearity can be achieved, and the testing method is applicable.
⑥ Reproducibility
Inoculate a certain amount of test bacteria in the sample (the inoculation amount should be above the limit of quantification), and use the pharmacopoeia method and the alternative method, respectively, by different personnel, at different times, using different reagents (or instruments) for inspection, and the inspection results are analyzed. Statistical analysis, relative standard deviation (RSD) was used to evaluate the difference in reproducibility between the two methods.
⑦ Durability
A comparison of the robustness of the alternative method to the pharmacopeia method is not required, but the robustness of the alternative method should be evaluated individually in order to understand the critical operating points of the method.
Check more quality microbiology test kit.